INTRODUCTION
In December 2019, an outbreak of a novel coronavirus-based disease was reported in Wuhan, China. The new virus, 2019-nCoV, so called then, was isolated on January 7, 2020, and identified as the cause of the outbreak (WHO report, 2020). The 2019-nCoV virus rapidly spread across China and many other countries and caused a rapidly growing global outbreak. On February 11, 2020, the World Health Organization (WHO) named this coronavirus “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2) and the disease that it caused “coronavirus disease 2019” (COVID-19) and on March 12, 2020, the total number of COVID-19 confirmed cases reached 125,260 globally with 80,981 cases in China and 44,279 outside of China and the COVID-19 was declared to be a pandemic by the WHO (WHO, COVID-19, 2020). As October 31, 2020, SARS-CoV-2 has affected over 216 countries, and about 46,394,009 cases have been confirmed around the world, of which 1,201,902 people have died.
CORONAVIRUS: THE PAST AND THE PRESENT
COVID-19 is caused by the SARS-CoV-2 that belongs to the beta-coronaviruses subfamily. Coronaviruses, a genus of the Coronaviridae family, are enveloped viruses with a large plus-strand ribonucleic acid (RNA) genome (Fehr et al., 2015). The genomic RNA is 27–32 kb in size, capped, and polyadenylated. Coronaviruses were identified in several non-human species, including rats, mice, chickens, cattle, turkeys, swine, cats, dogs, rabbits, and horses. In these species, coronavirus infection often causes devastating epizootics of respiratory or enteric diseases. Several coronaviruses, such as HCoV-229E and HCoV−OC43, were identified since the mid-1960s. Before the SARS-CoV outbreak, coronaviruses were only thought to cause mild, self-limiting respiratory infections in humans, commonly referred at as “colds.” These viruses are endemic among the human populations, causing 15–30% of respiratory tract infections each year. Rarely, these viruses can cause lower respiratory tract infections, especially in neonates, in the elderly, and in individuals with underlying illnesses.
SARS-CoV, a novel coronavirus, was identified in 2002 as the pathogenic agent of the SARS outbreak that occurred in in the Guangdong Province of China (Peiris et al., 2003). The virus showed phylogenetic similarities to both SARS-CoV and MERS-CoV viruses. However, SARS-CoV-2 displays a more efficient transmission pattern when compared with SARS-CoV and MERS-CoV (Liu et al., 2020), retaining a high transmission rate also in the asymptomatic incubation period (Lauer, 2020). So far, SARS is the most severe human disease caused by a coronavirus. Recent evidence confirmed that the SARS-CoV virus originated from a mutation occurring in a non-human host, probably bats, and gained the ability to affect humans. The virus was confirmed to spread through respiratory droplets from coughs or sneezes (Zhao et al., 2020; Wang et al., 2020; Chan et al., 2020) with the ability of the host to shed the infection while asymptomatic (Bai et al., 2020). Studies are now also proposing the possible feco-oral transmission of the virus (Gu et al., 2020).
On January 30, 2020, The WHO declared COVID-19 a public health emergency of international concern, and on March 11, the WHO Director General referred to COVID-19 as a pandemic. The epidemic has put public health systems under severe strain both in western countries and in the developing world.
The clinical spectrum of COVID-19 syndrome varies remarkably, going from asymptomatic forms to acute bilateral pneumonias requiring hospitalization. A significant percentage of cases requires admission to intensive-care-units (ICU) due to acute respiratory distress syndrome (ARDS) that requires mechanical ventilation support.
The newly emerging COVID-19 infection is accompanied by an aggressive inflammatory response with the release of a large amount of pro-inflammatory cytokines in an event known as “cytokine storm.” The host immune response to the SARS-CoV-2 virus is hyperactive resulting in an excessive inflammatory reaction. Several studies analyzing cytokine profiles from COVID-19 patients suggested that the cytokine storm (CS) correlated directly with lung injury, multi-organ failure, and unfavorable prognosis of severe COVID-19 (Huang et al., 2020; Ruan et al., 2020; Chen et al., 2020; Gao et al., 2020).
COVID-19 AND IMMUNE SYSTEM
The immune system has an exquisite mechanism capable of responding to various pathogens. Normal anti-viral immune response requires the activation of the inflammatory pathways of the immune system; however, aberrant or exaggerated response of the host’s immune system can cause severe disease if remains uncontrolled. Cytokines are an essential part of the inflammatory process. Cytokines are produced by several immune cells including the innate macrophages, dendritic cells, natural killer cells, and the adaptive T and B lymphocytes. During an innate immune response to a viral infection, pattern recognition receptors (PRRs) recognize different molecular structures that are characteristic to the invading virus. These molecular structures are referred to as pathogen associated molecular patterns (PAMPs). Binding of PAMPs to PRRs triggers the start of the inflammatory response against the invading virus resulting in the activation of several signaling pathways and subsequently transcription factors which induce the expression of genes responsible for production of several products involved in the host’s immune response to the virus, among which are the genes encoding several pro-inflammatory cytokines. The major transcription factors that are activated by PRRs are nuclear factor kB, activation protein 1, and interferon (IFN) response factors three and seven. These transcription factors induce the expression of genes encoding inflammatory cytokines, chemokines, and adhesion molecules. This sequence of events results in recruitment of leukocytes and plasma proteins to site of infection where they perform various effector functions that serve to combat the triggering infection (Thompson et al., 2011).
THE CS-INDUCED BY SARS-Cov-2 INFECTION
The term “cytokine storm” has become increasingly used not only by authors of scientific articles but also by popular media. CS characterize a wide spectrum of infectious and non-infectious diseases, and since 2005, it was associated to the avian H5N1 influenza virus infection (Yuen, 2005). Apart from the immediate significance of the term CS, the biological and clinical consequences of this immune system hyperactivity are by far less known, making it worthwhile to be briefly overviewed.
Experts said that CS have been observed in several individuals who are critical with COVID infection. A study named “COVID-19: Pathogenesis, CS and therapeutic potential of interferons” published in Elsevier Public Health Emergency Collection, accessible in PubMed Central repository of the National Institute of Health, USA, says that ARDS is the main cause of death in COVID-19 disease. It further states that one of the main features of ARDS is the CS – an uncontrolled systemic inflammatory response resulting from the release of pro-inflammatory cytokines by immune activated cells. As per the study, with high blood levels of cytokine in the body, it starts working inefficiently including the immune system.
Hence, here, the concept of a CS and the biological consequences of cytokine overproduction are reviewed. Furthermore, the CS through the lens of genomics is looked into, which is revealing the importance of the kinetics of cytokine gene expression and the remarkable degree of redundancy and overlap in cytokine signaling. Finally, it is discussed why some drugs such as Tocilizumab (TCZ), an IL-6 inhibitor is relatively effective and safe; besides corticosteroids, programmed cell death protein (PD)-1/PD-L1 checkpoint inhibition, cytokine-adsorption devices, intravenous immunoglobulin, and antimalarial agents in treating COVID-19 patients.
CYTOKINES
Cytokines are a diverse group of small proteins that are secreted by cells for the purpose of intercellular signaling and communication. Among the many functions of cytokines are the control of cell proliferation and differentiation and the regulation of angiogenesis and immune and inflammatory responses. The complex network of the cytokine response is best considered a series of overlapping networks, each with a degree of redundancy and with alternate pathways.
CYTOKINES ASSOCIATED WITH THE CS
IFN
The IFNs are a family of cytokines that play a central role in innate immunity to viruses and other microbial pathogens (Fensterl et al., 2009; Katze et al., 2008). They signal through the Jak-STAT signaling pathway. Receptor binding results in the initiation of downstream signaling cascades, the result of which is the activation of transcription factors and the induction of hundreds of IFN-stimulated genes. These genes encode protein products with antiviral, antiproliferative, or immunomodulatory properties.
Interleukins (ILs)
In contrast to the IFNs, the ILs are a diverse family of immune system regulators that function primarily in immune cell differentiation and activation. They may be either pro- or anti-inflammatory and, like all cytokines, elicit a wide variety of responses.
IL-1α and IL-1β are pro-inflammatory cytokines that mediate the host response to infection through both direct and indirect mechanisms (Dinarello, 2009).
IL-6 deserves a more extensive discussion in view of its involvement in the coronavirus-induced CS. IL-6 is crucially involved in acute inflammation due to its role in regulating the acute phase response (Brocker et al., 2010). It is produced by almost all stromal cells and B lymphocytes, T lymphocytes, macrophages, monocytes, dendritic cells, mast cells, and other non-lymphocytic cells, such as fibroblasts, endothelial cells, keratinocytes, glomerular Mesangial cells, and tumor cells. The production of this cytokine is increased by IL-1β and tumor necrosis factor (TNF)-α. IL-6 may also be responsible for the activation of T helper 17 (TH17) cells. In COVID-19 affected patients, a high TH17 cells activation could result from a virus-driven increased production of IL-6 by the immune system. IL-6 plays a key role in the pathogenesis of the CS due to its pleiotropic properties. Several studies showed that the serum levels of IL-6 are increased in COVID-19 patients (Zhang et al., 2020; Chen et al., 2020; McGonagle et al., 2020).
During the present COVID-19 pandemic, the use of TCZ as a therapeutic agent was proposed. By blocking the IL-6-receptor interaction, TCZ inhibits the IL-6-mediated signal transduction.
Chemokines
Chemokines are a large family of cytokines characterized by a powerful chemotactic effect. Chemokines act as chemo-attractants in the migration of the immune system cells, but they are also involved in several other processes including the development and function of innate and adaptive immune system, embryogenesis, and cancer metastasis (Rotondi et al., 2007). They are promptly secreted by a variety of cells in response to viral or microbial infections (Sozzani et al., 2000). Indeed, in a mouse model of IL-2 – induced ARDS, an upregulation of the mouse CXCL10 analog mob-1 mRNA was observed at initiation of lung injury (Neville et al., 1995). CXCL10 signaling appears to be a critical factor for the onset of ARDS, as shown in mice models of ARDS induced by either acid aspiration or by viral infection (with influenza H5N1 virus). Briefly, Ishikawa et al. (2012) demonstrated that wild-type mice developing ARDS had increased levels of CXCL10 mainly due to an increased secretion by infiltrating neutrophils, which induced an autocrine loop mechanism on the chemotaxis of inflamed neutrophils, leading to fulminant pulmonary inflammation.
THE INVOLVEMENT OF CHEMOKINES IN VIRAL INFECTIONS
Viral infections are associated with enhanced expression of several chemokines, in particular the IFNs-inducible ones. IFNs, which can be produced by any mammalian cell, are involved in the rapid and efficient host innate response against viruses. A powerful IFN response triggered by the first contact with a virus can slow down viral multiplication and “buy time” for the organism to establish a more efficient adaptive immune response (Haller et al., 2006). IFNs can stimulate surrounding cells to express potent antiviral proteins including enzymes, transcription factors, cell surface glycoproteins, cytokines, and chemokines. Moreover, they can inhibit cell proliferation, regulate apoptosis, and modulate the immune response (Weber et al., 2004). Among IFNs-induced molecules, the chemokine CXCL10 is currently regarded as a main player in the organism anti-viral response (Hayney et al., 2017), and particularly in respiratory tract infections. Several studies demonstrated that CXCL10 levels, as evaluated in serum, bronchial-alveolar washing fluid or nasal secretions, consistently correlate with the severity and duration of acute respiratory tract infection due to viral infections (Almansa et al., 2011). Furthermore, CXCL8 plays a major role in the initial control of respiratory tract infection due to its chemotactic activity for neutrophils and monocytes. CXCL8 levels in the nasal washing fluid correlate with symptoms severity during acute respiratory tract infections (Henriquez et al., 2015).
Most recently, several studies investigated the involvement of chemokines in coronavirus-related infective disease. It emerged that specific chemokines could play a crucial role in the development of COVID-19-related symptoms, thus confirming what previously known for other types of coronaviruses, such as SARS and MERS (Channappanavar and Perlman, 2017; Li et al., 2020). These findings could be somehow expected in view of the well-known role of chemokines in viral infections.
TNFs
TNF is perhaps the best known and most intensely studied of the pro-inflammatory cytokines, and it plays a prominent role in the CS literature. TNF is now considered a central cytokine in acute viral diseases, including those caused by influenza virus, dengue virus, and Ebola virus. To date, most studies have focused on direct measurements of a few cytokines and chemokines in the peripheral blood compartment and have failed to interrogate the whole of the immune cascade in the context of the infecting pathogen and the rapidly changing immune environment in tissues. Interestingly, while the peripheral blood may not provide an accurate picture of the cytokine profiles in a tissue, in the lungs, the location of the initial infection does not seem to be a determinant of the severity of local and systemic CS. For example, influenza viruses infect and destroy the ciliated epithelial cells of the conducting airways, whereas SARS-CoV infects type II pneumocytes in the alveolar walls, yet both can lead to indistinguishable clinical syndromes of acute lung injury with respiratory failure, sepsis, and a CS.
THE CS
The term “cytokine storm” calls up vivid images of an immune system gone awry and an inflammatory response flaring out of control. The term has captured the attention of the public and the scientific community alike and is increasingly being used in both the popular media and the scientific literature. However, while the general concept of an excessive or uncontrolled release of pro-inflammatory cytokines is well known, an actual definition of what constitutes a CS is lacking. Furthermore, there is not a good understanding of the molecular events that precipitate a CS, of the contribution such a “storm” makes to pathogenesis, or of what therapeutic strategies might be used to prevent the storm or quell it once it has started.
CS is a critical life-threating condition requiring intensive care admission and having a quite high mortality. CS is characterized by a clinical presentation of overwhelming systemic inflammation, hyperferritinemia, hemodynamic instability, and multi-organ failure, and if left untreated, it leads to death. The trigger for CS is an uncontrolled immune response resulting in continuous activation and expansion of immune cells, lymphocytes, and macrophages, which produce immense amounts of cytokines, resulting in a CS. The CS clinical findings are attributed to the action of the pro-inflammatory cytokines such as IL-1, IL-6, IL-18, IFN-γ, and TNF-α (Shimizu et al., 2019). CS has been reported in several viral infections including influenza H5N1 virus (Ishikawa et al., 2012; Kalaiyarasu et al., 2016), influenza H1N1 virus (Woo et al., 2010), and the two coronaviruses highly related to COVID-19; “SARS-CoV;” and “MERS-CoV.” Both pro-inflammatory cytokines (e.g., IL-1, IL-6, and TNF-α) and anti-inflammatory cytokines (e.g., IL-10 and IL-1 receptor antagonist) are elevated in the serum of CS patients. The main contributors to the interplay of the CS are IL-6 and TNF-α. In the absence of an immediate and appropriate therapeutic intervention, patients develop ARDS as a result of acute lung damage, followed by multi-organ failure and resulting in death. Hence, the CS should be treated immediately, otherwise mortality can result.
Since the first reports on COVID-19 disease, it appeared clear that ARDS accounted for a significant number of deaths among infected patients and that ARDS should be regarded as the hallmark immune-mediated clinical consequence in SARS-CoV-2 (Xu et al., 2020). ARDS is a devastating event, with an estimated mortality of approximately 40%, defined as the presence of bilateral lung infiltrates and severe hypoxemia. ARDS pathogenesis involves inflammatory injury to the alveolar capillary membrane, which results in increased lung permeability and the exudation of protein-rich pulmonary edema fluid into the airspaces, leading in the end to respiratory insufficiency (Bhatia et al., 2012). This concept originated from the observation that COVID-19 patients requiring ICU admission displayed higher concentrations of CXCL10, CCL2, and TNFα as compared to those in which the infection was less severe and did not require an ICU admission. To further complicate the issue, it should be highlighted that, in patients with SARS-Cov-2 infection, at difference from SARS-CoV infection, there is also an increased secretion Th2-immune-oriented cytokines such as IL-4 and IL-10, whose main effect is to suppress inflammation (Zhang et al., 2020; Cameron et al., 2008).
Taken together, studies show that, in SARS-CoV infection, ARDS is the ultimate result of a CS. In this scenario, the release by immune effector cells of large amounts of pro-inflammatory cytokines (IFNα, IFNγ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNFα, and TGFβ) and chemokines (CXCL10, CXCL8, CXCL9, CCL2, CCL3, and CCL5) precipitates and sustains the aberrant systemic inflammatory response. The CS is readily followed by the immune system “attacking” the body, which, in turn, will cause ARDS and multiple organ failure, the final result being death, at least in the most severe cases of SARS-CoV-2 infection (Xu et al., 2020).
GENOMIC VIEWS OF THE CS
One of the challenging clinical questions about the CS is why some individuals seem particularly susceptible yet others seem relatively resistant, and there has been a great deal of interest in identifying underlying genetic mechanisms. The compilation of cytokine and chemokine genomic data from influenza, SARS-CoV, and dengue studies provides important insight into our understanding of the CS. In particular, the dynamic transcriptional responses among the molecular components involved in cytokine and chemokine gene expression, including their kinetic properties and the timing of gene activation, are beginning to detail the events surrounding the CS.
CYTOKINE GENE EXPRESSION KINETICS
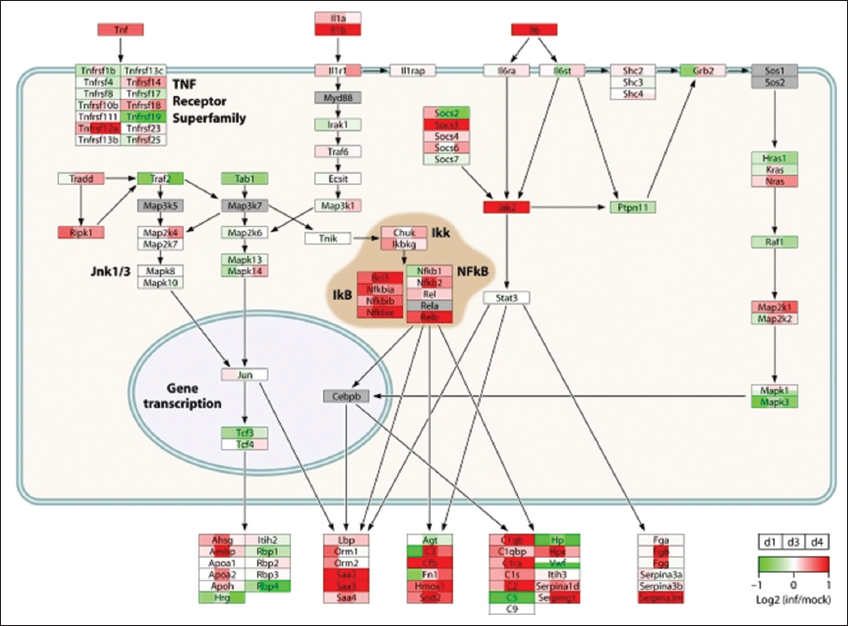
The paradigm of “hit hard and hit early” popularized for the treatment of AIDS also appears to apply to the way H5N1 avian influenza virus causes tissue damage in human infections. The rapid and intense nature of the host inflammatory response is the suspected cause of severe lung damage (Peiris et al., 2003). In a case study conducted in Ho Chi Minh City, Vietnam, comparing 18 people infected with H5N1 virus in 2004 and 2005 to 8 people infected with seasonal H1N1 influenza virus, elevated levels of MCP-1 (known as CCL2), IFN-γ-inducible IP-10 (CXCL10), MIG protein (CXCL9), and IL-8 were observed in H5N1 virus-infected patients who progressed to severe lung injury (de Jong, 2006). Over the past decade, compelling genomic evidence from animal model systems indicates that highly pathogenic influenza viruses aberrantly regulate cytokine and chemokine transcriptional responses, leading to a CS. The first genomic study to analyze host transcriptional responses to the 1918 pandemic influenza virus was led by Kash et al. (2006). Extensive lung damage in mice infected with 1918 virus was accompanied by highly upregulated cytokine and chemokine gene expression (Kash et al., 2006). Transcriptional activation of innate immune genes was observed as early as 1 day post-infection and remained sustained through the course of infection. These genomic data suggested that the host response enhances 1918 virus pathogenesis by initiating a CS that contributes to increased disease severity. Microarray analysis of lung tissue from macaques infected with H5N1 virus revealed prolonged expression of CCL2, CXCL10, and CXCL9 genes among a gene set of 45 significantly differentially expressed cytokine and chemokine genes. The strong IFN and inflammatory transcriptional responses early in infection combined with histopathological findings for H5N1-infected type II pneumocytes likely account for irreversible lung damage caused by inflammation. The network architecture for acute-phase molecular interactions and transcriptional responses to 1918, H5N1, and seasonal H1N1 virus infections is displayed in Figure 1.
Figure 1: The diagram shows transcriptional changes for cytokine signaling pathway molecules in mouse lungs infected with H5N1 virus. Ref: Jennifer, 2012
KEY CS MEDIATORS
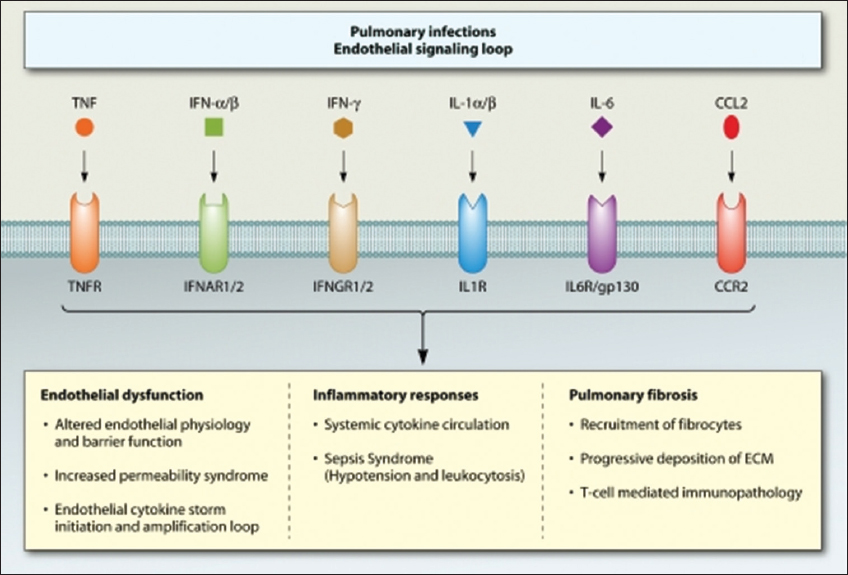
The functional role of cytokines during influenza virus infection has been pursued largely using single cytokine gene and cytokine receptor gene knockout (KO) mice, including those lacking IL-6 (Szretter et al., 2007), IL-17RA (Crowe et al., 2009), or CCR2 (Dessing, 2007), or using KO mice lacking multiple cytokine receptor genes, such as IL-1R and TNFR (Perrone et al., 2010). Transgenic mice with humanized immune systems present a greater opportunity to study cytokines in a more clinically relevant animal model. A combination of KO mouse models and pharmacological intervention (administration of chemokine receptor small-molecule antagonists and/or cytokine antibodies) will likely provide greater insight into the CS and the redundancy that may exacerbate disease [Figure 2]. It shows the functional roles of key cytokines and chemokines and their cognate receptors in the development of main clinical outcomes associated with the CS.
Figure 2: Role of key cytokines and their cognate receptors
CAN PREVENTING CS EASE SEVERE COVID-19 SYMPTOMS?
The early recognition of CS and the prompt treatment can lead to better outcome. Several biological agents targeting cytokines have been proposed for treating CS. TCZ is a recombinant humanized IL-6 receptor antagonist that interferes with IL-6 binding to its receptor and blocks signaling. TCZ is used in the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, and giant cell arteritis and has proven valuable in treatment of CS. Downstream inhibitors of cytokines, for example, JAK inhibitors, are also being explored in treating CS. There is no way to prevent CS, it is not possible to stop cytokine from working because, in the absence of cytokine, the virus itself will kill the patient. The storm can only be modulated through immunomodulators that can keep the immune system active and at the same time not attacking all cells. Various drugs, such as anti-tumor necrosis factor and cyclooxygenase inhibitors, are potential CS inhibitors. Some of them are currently in clinical trials for COVID-19.
Collectively, the clinical, immunological, and pathologic features of COVID-19 have something in common with SARS and MERS. For example, all the viruses can cause lymphopenia and influenza-like symptoms in the early stage. SARS and COVID-19 do not lead to the upgrade of TNF-α, but the increase of IL-6 and IL-10 is more prevalent in COVID-19. The IL-6 plays a crucial role in the pathology of COVID-19, including the chemotaxis of neutrophils and lymphocyte necrosis. Importantly, COVID-19 is more able to cause cytotoxic lymphocytes exhaustion.
POTENTIAL TREATMENTS FOR CS IN COVID-19 AND THEIR SAFETY
IL-6 Inhibition
SARS-CoV-2 mainly causes a dramatic increase in IL-6 and does not remarkably promote other pro-inflammatory factors, such as IL-1β and IFN-γ. TCZ is a recombinant humanized anti-human IL-6 receptor monoclonal antibody, preventing IL-6 binding to its receptor to exert the immunosuppression promoted by IL-6 (Michot et al., 2020; Zhang et al., 2020). All the cytokines produced by immune cells are responsible for viral clearance. Suppression of cytokine release at an early stage of disease as treatment is controversial. The timing and the doses of the intervention still need to be inspected clearly. Encouraging results have been reported in China where TCZ was used in treatment of 21 patients with severe and critical COVID-19. Clinical data showed that the symptoms, hypoxygenmia, and CT opacity changes were improved immediately after the treatment with TCZ in most of the patients, suggesting that TCZ could be an efficient therapeutic agent for treatment of the CS associated with COVID.
CORTICOSTEROIDS
Glucocorticoids reduce the proliferation, activation, differentiation, and survival of T cells and macrophages. Glucocorticoids offer inhibitory actions on the transcription and action of various cytokines. The Th1 and macrophage-based pro-inflammatory cytokines IL-1β, IL-2, IL-6, TNF-α, and IL-17 are inhibited by glucocorticoids. Glucocorticoid therapy is used widely among critically ill patients with other coronavirus infections (e.g., SARS and MERS). Corticosteroids have been administered to ICU patients infected with SARS-CoV-2 (Wang et al., 2020; Guan et al., 2020).
CYTOKINE ABSORPTION DEVICE
Cytokine adsorption involves using a method, such as extracorporeal membrane oxygenation, to filter harmful substances directly. An extracorporeal cytokine hemoadsorption device called Cytosorb® (Cytosorbents, Monmouth, NJ, USA) has been reported to capture and reduce inflammatory mediators. Neutralizing excessive cytokines with hemoadsorption devices might be relatively effective. The disadvantage is like corticosteroids: A wide range of cytokines would be adsorbed. Thus, it would lead to a lack of cytokines, which are at reasonable or even insufficient levels. Hence, it is suggested that treating the CS in COVID-19 should be based on the laboratory results of cytokines and chemokines.
HYDROXYCHLOROQUINES
Despite a lack of clinical evidence, the US gave emergency approval to HCQ, a member of antimalarial agents, in COVID-19 on March 28 (Lenzer et al., 2020). A meta-analysis included the studies up to April 5, 2020 (Singh, 2020) and showed that four clinical trials and three observational studies are eligible for the study. Unfortunately, the authors concluded that HCQ has no clinical effect on patients with COVID-19. Low-dosage of HCQ could be beneficial for critically ill patients with COVID-19. The study also indicates high dosage HCQ might not be suitable for critically ill patients because of its potential safety hazards.
CONCLUSIVE REMARKS
The elevations of IL-6 and IL-10 are highly consistent in COVID-19. IL-6 targets the IL-6 receptor, and the letter recruit JAK, which transit cascade signal to activate signal transducer and activator of transcription 3 (Tanaka et al., 2014). A precise definition of a CS is needed urgently. Mehta et al. suggested that the criteria of sHLH could be applied. Moreover, the term needs to be placed in the ICD code. The ICD code would bring the standardization of disease names, the convenience of electronic medical records management, and the efficiency in information sharing. For example, the characteristic of CS would be more accessible to be collected for a retrospective study. SARS-CoV-2 and its related syndrome COVID-19 have been known to the scientific community since <5 months. Clearly much is yet to be understood, and the challenge for the next future will be to increase our understanding the physiopathology of this novel infectious disease. Hopefully, the advances in our comprehension of the mechanisms sustaining the clinical course and patients-related factors driving the final outcome will be helpful in developing effective preventive strategies and/or therapeutical options. Based on current knowledge, the “cytokine storm” appears as one of the most dangerous and potentially life-threatening event related to COVID-19 sustaining its major clinical consequences. The immune mediated events related to the response to SARS-CoV-2 infection, and the role of the chemokine/chemokine receptor system will be further and more extensively characterized with the final goal to identify targeted therapeutic strategies. Although lessons from the previous SARS and MERS epidemics can be drawn, there is still much to do in order to conclude whether SARS-CoV-2 virus behaves in the same way of its predecessors or if it is characterized by peculiar specificities. Clearly, the hide-and-seek challenge between the virus and our immune defense will also help us understanding the extremely variable spectrum of clinical manifestations of COVID-19, which appears to range between asymptomatic cases to possibly lethal bilateral pneumonia with multi-organ failure.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.