Paul et.al.
deposits(Anthony etal. ,2003).Asbondstooxygenand
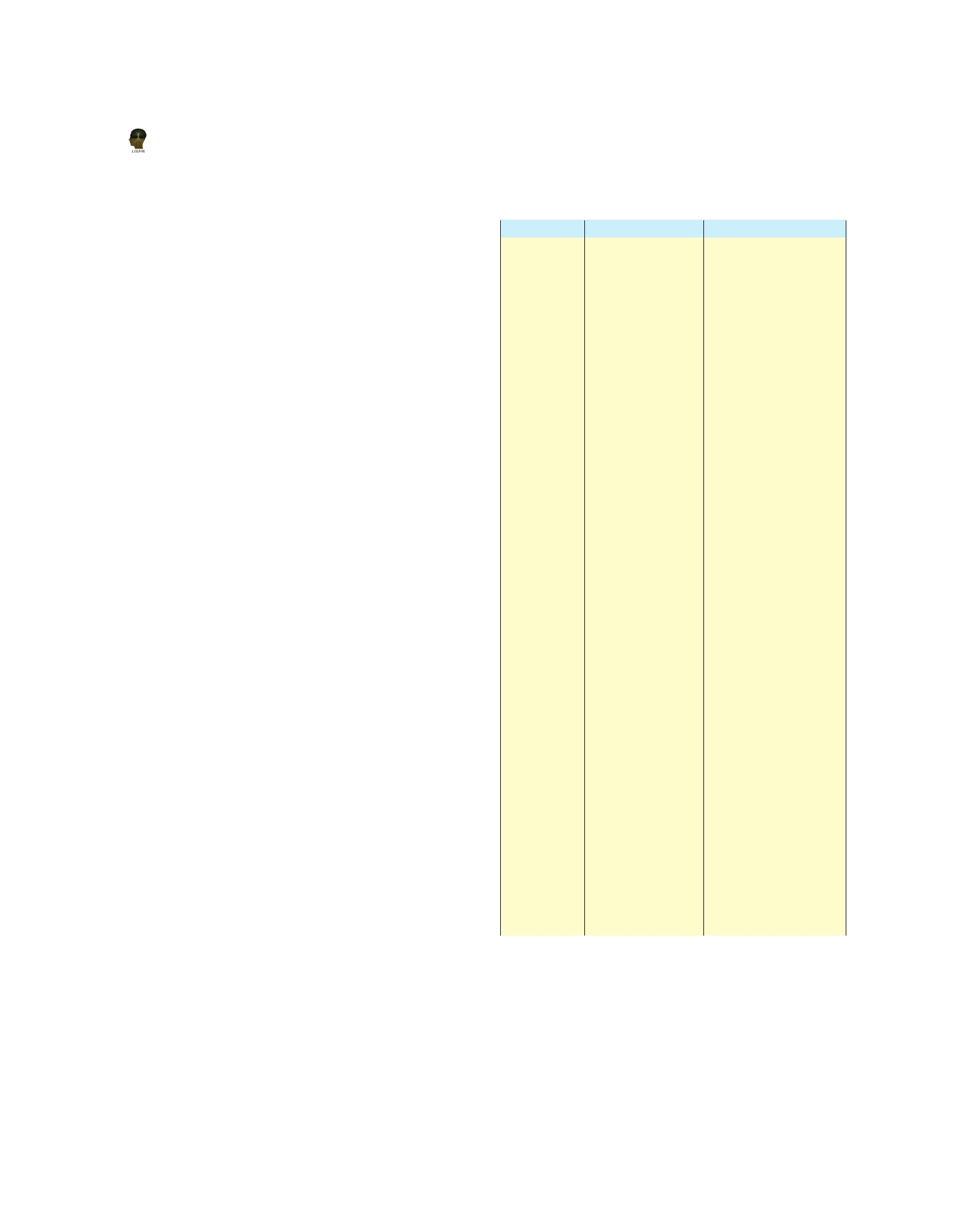
Table 1. Major As mineral occurring in nature (Smedley
sulphur to produce a multitude of aqueous species
and Kinniburgh 2002)
and minerals like Arsenolite (As 2 O 3 ), Arsenopyrite
Mineral
Composition
Occurrence
(FeAsS), Realger (As S ), Orpiment (As S ) etc (O’Day
4 4
2 3
Native As
As
Hydrothermal veins
2006). Moreover, via biomethylation certain groups
Niccolite
NiAs
Vein deposits and norites
of microorganisms produce a large number of
Vein
deposits,
often
organoarsenic compounds (Stolz et al. , 2006), namely
associated
with
dimethylarsinate (DMA) and monomethylarsonate
Realgar
AsS
orpiment,
clays
and
(MMA), generated by replacing a hydroxyl (-OH)
limestones, also deposits
from hot springs
ligand by a methyl group (-CH 3 ) in inorganic As (III)
and As (V) structures (Cullen and Reimer 1989).
Hydrothermal
veins,
Orpiment
As S
2 3
hot
springs,
volcanic
Redox potential (Eh) and pH are the main drivers
sublimation product
of As speciation in natural water, where the
H i g h - t e m p e r a t u r e
predominant chemical forms include oxyanions of
Cobaltite
CoAsS
deposits,
metamorphic
As (III) (H 3 AsO ) - or H 2 AsO -) and As (V) (H AsO
4 2
3
2
4-
rocks
or HAsO 4 2- ) (Suttigarn and Wang 2005; Rhine et al. ,
The most abundant As
2006). Generally, As (III) occurs in anoxic condition,
Arsenopyrite FeAsS
mineral,
dominantly
exhibiting greater toxicity and mobility than As (V)
mineral veins
which mainly occurs in aerobic conditions (Clifford
Tennantite
(Cu,Fe) 1 2
Hydrothermal veins
1990; Ehrlich 1996). Relative to the other oxyanion-
As4S 1 3
forming elements (e.g. Se, Sb, Mo, V, Cr, U, Re), As
Enargite
Cu 3 AsS 4
Hydrothermal veins
is the most problematic in the environment because
Secondary
mineral
of its relative mobility over a wide range of redox
formed by oxidation of
arsenopyrite, native
conditions (Smedley and Kinniburgh 2002).
Arsenolite
As 2 O 3
Arsenic and other As
Origin and sources of As
minerals
Secondary
mineral
Arsenic is a ubiquitous element in the environment. It
formed by oxidation of
ranks 20th in earth’s crust, 14th in sea water and 12th
Claudetite
As 2 O 3
realgar, arsenopyrite and
in the human body as an element (Woolson 1975). A
other As minerals
range of As compounds, both organic and inorganic,
Scorodite
FeAsO 4 .2H 2 O
Secondary mineral
are introduced into the environment through natural
and anthropogenic sources.
(Ni,Co)3
Annabergite
Secondary mineral
(AsO ) 2 .8H O
4
2
Natural Sources
Secondary
mineral,
Hoernesite
Mg 3 (AsO 4 ) 2 .8H 2 O
smelter wastes
In nature, As occurs rarely in its elemental form
Conichalcite
CaCu(AsO 4 )(OH)
Secondary mineral
and is widely distributed in a variety of minerals,
Oxidation product of
commonly as arsenide of iron, copper, lead, silver
P h a r m a c o - Fe 3 (AsO 4 )
arsenopyrite and other
siderite
and gold or as sulphide minerals, for example
2(OH) 3 .5H O
2
As minerals
arsenopyrite (O’Day 2006). Weathering of these
The greatest concentrations of these minerals occur in
As bearing minerals, volcanic activity as well
mineralised areas and are found in close association
as mining waste resulted in occurrence of As in
with the transition metals as well as Cd, Pb, Ag, Au,
aquatic and terrestrial environment under both
Sb, P, W and Mo (Smedley and Kinniburgh 2002).
oxic and anoxic condition (Rhine et al. , 2006). A list
Oxidation and dissolution of the most common As
of some of the most common As minerals along
bearing minerals such as arsenian pyrite [Fe(AsS) ],
2
with their place of occurrence is given in Table 1.
190